Lessons
Types of Chemical Reactions
3) Displacement Reaction
4) Double Displacement Reaction,
Difference between Displacement & Double Displacement Reaction,
5) Redox Reaction = Oxidation + Reduction
3) Displacement Reaction
4) Double Displacement Reaction,
Difference between Displacement & Double Displacement Reaction,
5) Redox Reaction = Oxidation + Reduction
1-PART-3
Effect of Oxidation reaction in everyday life.
Corrosion & Prevention
Rancidity & Prevention
Corrosion & Rancidity Difference
Corrosion & Prevention
Rancidity & Prevention
Corrosion & Rancidity Difference
1-PART-4
1-TEST-01-08-2021
Acid Base & Salt Defination With Examples
Acide & Base Difference
Indicators Defination & Acid Base Indicators - Litmus / Phenolpthalein / Methyl Orange / Olfactory indicators
Aqueous Solution(water) & Alkali (water soluble base)
Acide & Base Difference
Indicators Defination & Acid Base Indicators - Litmus / Phenolpthalein / Methyl Orange / Olfactory indicators
Aqueous Solution(water) & Alkali (water soluble base)
2-PART-1
Chemical Properties of Acids
1) Metal + Acid ---> Salt + H2(g)
Displacement Reaction
2) Metal Carbonate or Metal Hydrogen Carbonate + Acid -> Salt + H2O(l) + CO2(g)
3) Acid + Base ---> Salt + H2O(l)
Double Displacement Reaction
Neutralization Reaction
4) Non-metallic Oxide + Base ---> Salt + H2O(l)
Non-metallic Oxide acts as Acidic Oxide or Acidic in Nature
5) Acid + Metal Oxide -> Salt + H2O(l)
Double Displacement Reaction
Metal Oxide are Basic in nature
1) Metal + Acid ---> Salt + H2(g)
Displacement Reaction
2) Metal Carbonate or Metal Hydrogen Carbonate + Acid -> Salt + H2O(l) + CO2(g)
3) Acid + Base ---> Salt + H2O(l)
Double Displacement Reaction
Neutralization Reaction
4) Non-metallic Oxide + Base ---> Salt + H2O(l)
Non-metallic Oxide acts as Acidic Oxide or Acidic in Nature
5) Acid + Metal Oxide -> Salt + H2O(l)
Double Displacement Reaction
Metal Oxide are Basic in nature
2-PART-2
Activity1: Learn Electrolytes & Non Electrolytes with help of activity
Electrolytes are of two types
Activity Conculsion: Ions must be Present in solution for it to conduct electricity
Activity2: Acidic Nature of Solutons
When solution of NaCl & Sulphuric acid are mixed hydrogen chloride gas is produced.
NaCl + H2SO4 --> Na2SO4 + HCL
This HCL gas is allowed to pass over
Dry blue litmus - does not turn red
Wet blue litmas - turns red
Activity Conculsion: Acids shows their acidic property (turning blue litmus red) only in presence of water
In presence of water, H+ ions are seperated from acid molecules
HCl(g) + H2O(l) --> H(aq)+ + Cl(aq)-
H+ ions cannot exists alone. They combine with water molecules to form hydonium ions. (H3O+)
H(aq)+ + H2O(l) --> H3O+
Acid are substances that produces H+ ions or H3O+ ions
Simillary Alkali's base produce OH- ions in aqueous solution
| Electrolytes | Non Electrolytes |
|---|---|
| Solution Which conduct Electricity | Solution Which do not conduct Electricity |
| Electrolytes contains ions | Non Electrolytes do not contain ions |
| Good conductor of electricity | Do not conduct or bad conductor of electricity |
| ex. NaCl Solution ,Dilute HCL ,Rain Water , Tab Water ,NAOH | ex. Gulcose Solution Alcohol Solution Distill Water |
| Strong Electrolytes | Weak Electrolytes |
|---|---|
| Electrolytes that contain many ions are called strong electrolytes | Electrolytes that contain few ions are called weak electrolytes |
| ex. NaCl, HCL, NAOH | ex. acetic acid, carbonic acid |
Activity2: Acidic Nature of Solutons
When solution of NaCl & Sulphuric acid are mixed hydrogen chloride gas is produced.
NaCl + H2SO4 --> Na2SO4 + HCL
This HCL gas is allowed to pass over
Dry blue litmus - does not turn red
Wet blue litmas - turns red
Activity Conculsion: Acids shows their acidic property (turning blue litmus red) only in presence of water
In presence of water, H+ ions are seperated from acid molecules
HCl(g) + H2O(l) --> H(aq)+ + Cl(aq)-
H+ ions cannot exists alone. They combine with water molecules to form hydonium ions. (H3O+)
H(aq)+ + H2O(l) --> H3O+
Acid are substances that produces H+ ions or H3O+ ions
Simillary Alkali's base produce OH- ions in aqueous solution
2-PART-3
Dilution & Method to dilute an acid, Strength of Acids or Bases, pH AND pH SCALE
Dilution - is the process in which an acid or base is mixed with water.
Dilution results in decrese in number of ions (H+ or OH-) per unit volume
Method to dilute an acid - Add acid to water dropwise with constant stirring
This is because (give reason)
1)Reaction of acid with water is exothermic & can cause acid mixture to splash out, causing acid burns
2)Due to local heating glass beaker can break causing accidents in laboratory
Strength of Acids or Bases - depends on number of H+ ions or OH- ions in solution
Universal indicators are mixtues of serveral indicators & shows different colors when the concentration of H+ ions are different.
To find the strength of acid or base, we can use Universal Indicators. ex.pH paper
Universal indicator are better then acid-base indicators (give reason)
beacuse they help to find the strenght of acid.
pH scale:is a scale to measure the H+ ions concentation in solution.
More the H+ ions lower the pH value.
pH: pH is Number which indicates if a solution is acidic or basic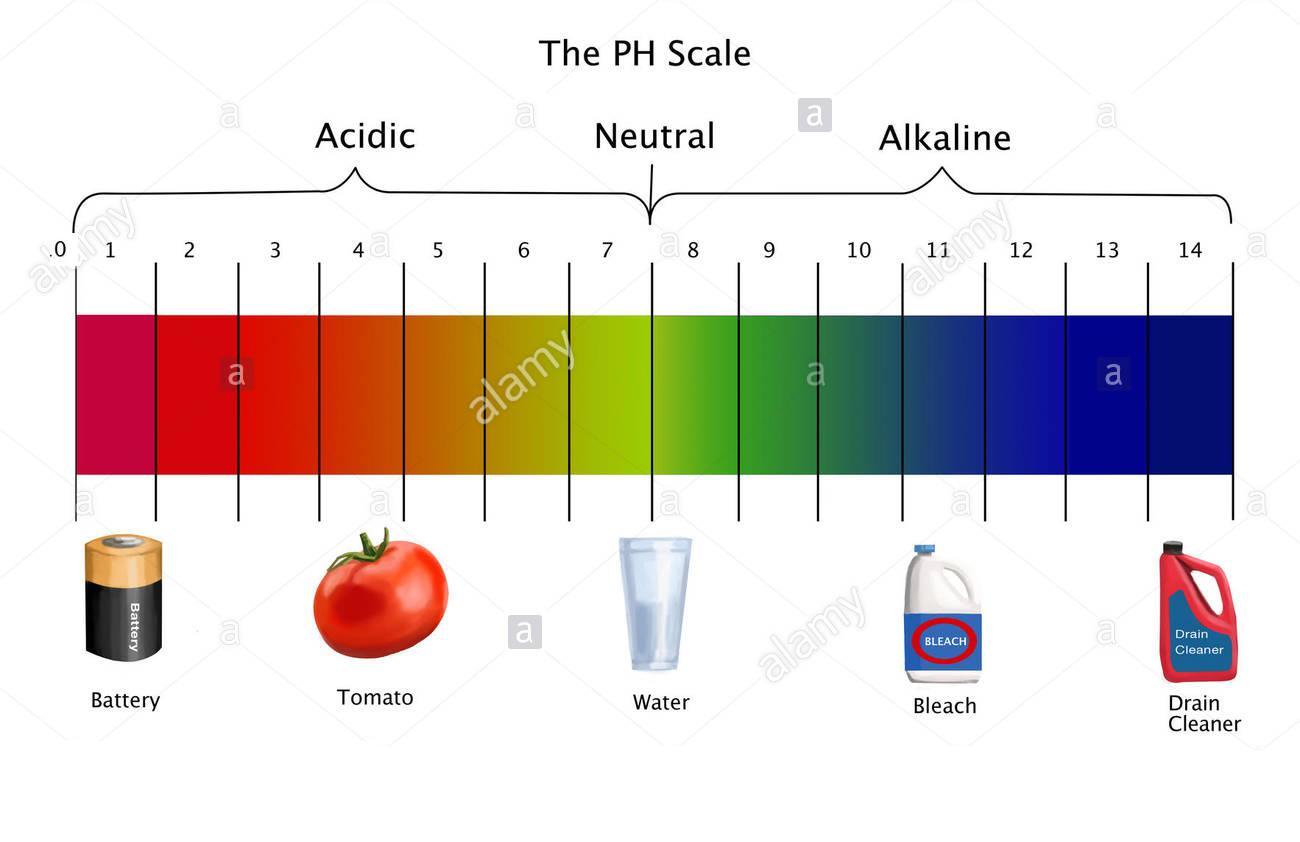
Dilution - is the process in which an acid or base is mixed with water.
Dilution results in decrese in number of ions (H+ or OH-) per unit volume
Method to dilute an acid - Add acid to water dropwise with constant stirring
This is because (give reason)
1)Reaction of acid with water is exothermic & can cause acid mixture to splash out, causing acid burns
2)Due to local heating glass beaker can break causing accidents in laboratory
Strength of Acids or Bases - depends on number of H+ ions or OH- ions in solution
| Strong acid or base | Weak acid or base |
| Are those susbstance which produces large number of ions in solution | Are those susbstance which produces less/few number of ions in solution |
| Strong acid ex. HCL, H2SO4, HNO3 | Weak acid ex citric acid, lactic acid, acetic acid, carbonic acid, phosphorice acid. |
| Strong base ex NAOH, KOH, CA(OH)2 | Weak base ex Mg(OH)2, NH4OH, AL(OH)3 |
| Strong bases are highely corrosive | We can consume Weak acid |
Universal indicators are mixtues of serveral indicators & shows different colors when the concentration of H+ ions are different.
To find the strength of acid or base, we can use Universal Indicators. ex.pH paper
Universal indicator are better then acid-base indicators (give reason)
beacuse they help to find the strenght of acid.
pH scale:is a scale to measure the H+ ions concentation in solution.
More the H+ ions lower the pH value.
pH: pH is Number which indicates if a solution is acidic or basic
- pH = 7 neutral solution
- pH < 7 acidic solution
- pH > 7 alkali solution

2-PART-4
Importance of pH in everyday life
- Are plants and animals pH sensitive
- What is pH of soil
- pH of our digestive
- pH change as cause of tooth decay
- Self defence by animal & plants
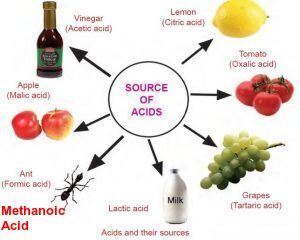
| Natural Source | Acid |
| Vinegar | acetic acid |
| Orange, lemon | citrc acid |
| Tamarind | Tartaric acid |
| Sour milk | lactic acid |
| Tomato | oxalic acid |
| Ant sting, Nettle Sting | Methanoic acid |
 |
|
2-PART-5
More about Salts
Family of Salts
pH OF SALT SOLUTIONS
Salt Defination
Family Of Salts Defination - Salt with same cation or same anion are said to beloung to same family.
Chemicals from Common Salt - Sea water contains many salts. NaCl can be seperated from these salts
Deposit of NaCl can be found inside this earths as brown crystals called rock salt.
Rock Salt is brown due to impurities present in it
Rock Salt is mined from earth.
Common Salt can be prepared by neutralising HCL & NAOH
HCL + NAOH ---> NaCl + H2O
Common salt is raw material for many-substances that we use in daily life.
NAOH - Sodium Hydroxide
It is prepared by the action of Cl2(g) on dry slaked lime
Cl2 + Ca(OH)2 --> CaOCl2 + H2O
Used
Washing Soda
Family of Salts
pH OF SALT SOLUTIONS
Salt Defination
Family Of Salts Defination - Salt with same cation or same anion are said to beloung to same family.
- Family of chloride - KCl, NaCl, CaCl2
- Family of carbonate - CaCO3, Na2CO3, ZnCO3
- Family of Sodium - NaCl, NaNO3, Na2CO3
- Family of calcium - CaCl2, Ca(NO3)2, CaCO3
| Strong acid | Strong base |
|
|
| Weak acid | Waek base |
|
|
- Salts of strong acids & strong base are neutral. ex. NaCl, KI, KNO3 (pH = 7)
- Salts of strong base & weak acid are alkaline ex. Na2CO3, NaHCO3, CH3COONa Sodium acetate (pH > 7)
- Salts of strong acids & weak base are acidic ex.CaSO4, NH4Cl ex.(pH < 7)
Chemicals from Common Salt - Sea water contains many salts. NaCl can be seperated from these salts
Deposit of NaCl can be found inside this earths as brown crystals called rock salt.
Rock Salt is brown due to impurities present in it
Rock Salt is mined from earth.
Common Salt can be prepared by neutralising HCL & NAOH
HCL + NAOH ---> NaCl + H2O
Common salt is raw material for many-substances that we use in daily life.
NAOH - Sodium Hydroxide
- Sodium Hydroxide is obtained by chlor-alkali process
- Chlor-alkali process is a process in whihc electricity is passed through brine (aqueous solution of NaCl)
- When current is passed, solution of NaCl decomposes to form H2(g) at cathode & Cl2 gas at anode and soluton of NaOH(aq) near cathod
- NaCl + H2O ----electricity----> H2 + Cl2 + NaOH
It is prepared by the action of Cl2(g) on dry slaked lime
Cl2 + Ca(OH)2 --> CaOCl2 + H2O
Used
- Bleaching Powder
- Oxidising agent
- Disinfect water
Washing Soda
2-PART-6
Water of Crystalisation
Water of Crystalisation: It is the fixed number of water molecules present in one formula unit of salt.
Ex.of Hydrated Salt
Plater of paris will abosrbs moisture from air and absorns water molecules equal to water of crystallisation and solidifies into a hard mass of gypsum. Hence POP is stored in air tight container or not exposed to moist air
Qestion Why to doctor use Plater of paris to set fracture bones in place
When Plater of paris is mixed with adiquite amount of water it gains water of crystallisation & solidifies into hard mass of gypsum.
CaSo4.1/2H2O + 1.1/2H2O --> CaSO4.2H2O
Water of Crystalisation: It is the fixed number of water molecules present in one formula unit of salt.
Ex.of Hydrated Salt
- CuSO4.5H2O - Blue
- FeSO4.7H2O - Green
- Na2CO3.10H2O - Washing Soda
- CaSO4.2H2O - Gypsum
- CuSO4.1/2H2O - Plater of Paris
- Water droplets appears on the cooler sides of testtube. Inferance:
- This indicates the crystals of CuSO4 contains water molecules (water of crystallisation)
- Blue crystal turns white
- Crystal turns in to white powder
- White powder turns into blue crystal
- Water of crystallisation is responible for the blue color of crystals & for its crystaline nature
- Water of crystallization is lost on heating and absorbed by crystal when adiquite amount of water is added.
- Used by doctors to set fracture bones in place
- Toys, statutes, material for decoration
- To make surface smooth
It is prepared by heating gypsum at 373K, it loosed its water of crystallisation and turns in to POP
CaSO4.2H2O --> CaSo4.1/2H2O + 1.1/2H2O
In POP, 2 units of salt and one molecule of H20 Heat Gypsum --> Plaster of Paris --> Add adiquite amount of water --> Gypsum back. Used of Plater of Paris
Plater of paris will abosrbs moisture from air and absorns water molecules equal to water of crystallisation and solidifies into a hard mass of gypsum. Hence POP is stored in air tight container or not exposed to moist air
Qestion Why to doctor use Plater of paris to set fracture bones in place
When Plater of paris is mixed with adiquite amount of water it gains water of crystallisation & solidifies into hard mass of gypsum.
CaSo4.1/2H2O + 1.1/2H2O --> CaSO4.2H2O
2-PART-7
Physical properties of metals
Metal and Non-Metal Difference
Allotropes Defination with ex.
Metal and Non-Metal Difference
Allotropes Defination with ex.
3-PART-1
Chemical Properties Of Metals
1) Reaction with air - Metal + Oxygen -> MetalOxide
3) Reaction with acid - Metal + Acid -> Salt + H2
4) Metal displacement reaction
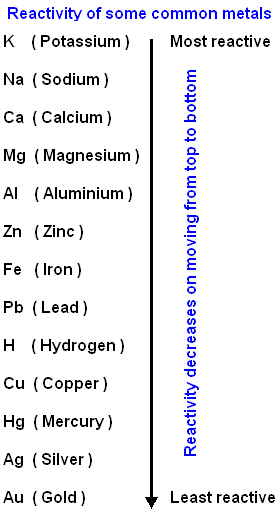
5) Reactivity Series or Activity Series Defination
1) Reaction with air - Metal + Oxygen -> MetalOxide
- Metal Oxide are basic & insoluble in water. Alkali is water soluble base
- Amphoteric Oxide - reacts with both acid and base to form salt & water
eg. Al2O3, ZnO
3) Reaction with acid - Metal + Acid -> Salt + H2
4) Metal displacement reaction
5) Reactivity Series or Activity Series Defination
|
|
3-PART-2
Electrovalent Compound or Ionic Compound - Compounds formed by transfer of electrons from metal to non-metal
Properties of Ionic Compound
Properties of Ionic Compound
- Ionic Compound are hard - Give Reason - there exists strong force of attraction between the ions
- Ionic Solids have high melting points - Give Reason
- Ionic Compound dissolve in water but do not dissolve in kerosine or petrol
- Ionic Compound are poor conductor in solid state - Give Reason
- Ionic Compound are good conductor of electricity in aqueous solution - Give Reason
- Ionic Compound are good conductor of electricity in molten state - Give Reason
3-PART-3
Occurence of Metals
Minerals & Ore Difference
Metallurgy Defination - Process of extraction of metal from its ore
1) Enrichment of ore
2) The ore is converted in to oxide form by process of Roasting or Calcination
3) Reduction of ore
Gangue Defination
Roasting & Calcination Difference
Minerals & Ore Difference
Metallurgy Defination - Process of extraction of metal from its ore
- Metals at the bottom of activity series which are least reactive are found in free state in nature
- Highly reactive metals are never found in free state in nature
- Moderate reactive elements like Zn, Fe, Pb, are found in the form of Oxides, sulphides or carbonates
- Oxide ore are more common because oxygen is a very reactive element & combines with all elements & it is abundant in nature
1) Enrichment of ore
2) The ore is converted in to oxide form by process of Roasting or Calcination
3) Reduction of ore
- Metals which are highely reactive like - K, Na, Mg, Ca, Al are reduced by electrolysis of molten ore
- Moderate reactive metals - Zn, Fe which are highely reactive are obtain by reducing their ores by reducing agents like Coke(C) or metal
- Least reactive metals - Ag, Hg are obtained by thermal decomposition of ore
Gangue Defination
Roasting & Calcination Difference
3-PART-4
Corresion - Oxidation of metal in presence of air & moisture
Examples of Corrision
Examples of Corrision
- Iron exposed to air - turns into brown flaky substance called rust
- Silver articles becoms black due to reaction with sulphur forming silver sulphide
- Copper reacts with moist CO2 in presence of air & form green coat of copper carbonate
- Oling , greasing, painting
- Galvanisation -coating iron articles with a layer of Zn.
Note:- Zn is more reactive then Iron Hence Protects iron from rusting even if coating is broken - Anodising - Coating with aluminium oxide
- Chrome Plating
3-PART-6
Bonding in Carbon - Carbon has Atomic number 6, electronic configuration (2,4)
- Gain 4e - -- If carbon gains 4e - then it forms anion ( C-4 ) with 6 protons and 10 electrons. and it is difficult for 6 protons to hold 10 electrons
- Loose 4e - -- If carbon looses 4e - then it forms cation ( C+4 ). But a large amount of energy is required to remove 4e - from its outermost shell. Hence carbon cannot loose 4 4e -
- Carbon attains noble gas configration by sharing electrons ( e - ) with other elements.
- Covalent bond defination
- Difference beterrn Electrovalent or Ionic compounds & Covalent compounds
4-PART-1
Covalent compounds Defination
Types of covalent Bounds
Types of covalent Bounds
- Single covalent bond
- Double covalent bond
- Triple covalent bond
- Hydrogen molecule - H2
- Oxygen molecule - O2
- Nitrogen molecule - N2
- Chlorine molecule - Cl2
- Amonia molecule - NH3
- Methane molecule - CH4
4-PART-2
Allotropes of Carbon defination
Carbon Allotropes Examples
Versalite nature of Carbon
Carbon Allotropes Examples
- Diamond
- Graphide
- Coal, Charcoal, Coke
- Fullerene
Versalite nature of Carbon
- Catenation - is the ability of carbon atom to form bonds with carbon atom. Due to Catenation, carbon atoms can form Long carbon chains,Branched chain or rings of carbon atoms
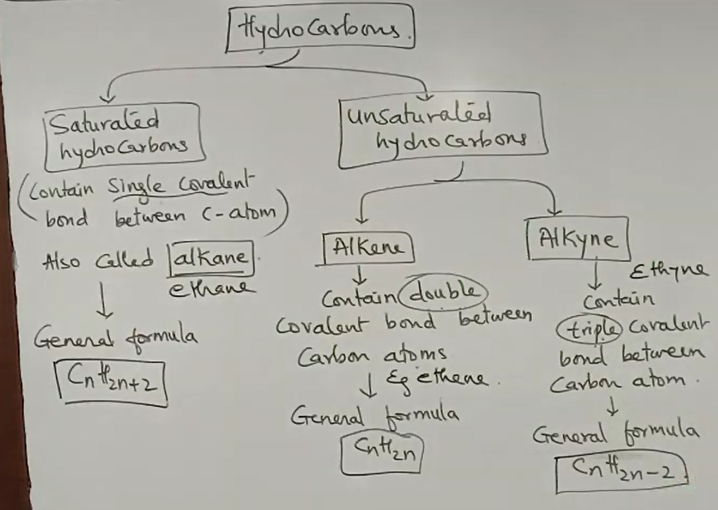
- Saturated Compounds - Compounds of carbocn with single covalent bond
- UnSaturated Compounds - Compounds of carbocn with double covalent bond or triple covalent bond
- Tetravalency - Carbon has 4e- in last shell, which it can share with carbon atoms or other monovalent atoms.
Note: carbon can also bond with oxygen, suplher, chlorine, nitrogen
4-PART-3-AND-4
Hydrocarbons - are compounds containing only hydrogen and carbon atoms in the molecule.
ex. CH4
Draw for the following for the Hydrocarbons given in table
Isomers - are compounds having same molecular formula but different structural formula.
Ring hydrocarbon
ex. CH4

Draw for the following for the Hydrocarbons given in table
- Molecular Formula
- Structural Formula
- Condensed Formula
| Hydrocarbons | |||
| n | Alkane CnH2n+2 |
Alkene CnH2n |
Alkyne CnH2n-2 |
| 1 | Methane | - | - |
| 2 | Ethane | Ethene | Ethyne |
| 3 | Propane | Propene | Propyne |
| 4 | Butane | Butene | Butyne e |
| n-butane iso-butane |
n-butene iso-butene |
n-butyne iso-butyne |
|
| 5 | Pentane | Pentene | Pentyne |
| n-pentane iso-pentane neo-pentane |
n-pentene iso-pentene neo-pentene |
n-pentyne iso-pentyne neo-pentyne |
|
Isomers - are compounds having same molecular formula but different structural formula.
Ring hydrocarbon
- Cyclohexane
- Benzene
4-PART-5-AND-6
Heteroatom - is an element that replaces H-atom (hydrogen atom) in hydrocarbon.
Cl, N, O, S
Functional Group - is a heteroatom or group containing heteroatom which gives specific properties to compound, regardless of length and nature of carbon chain
Homologous compound - is a series of compounds in which same functional group substitutes for hydrogen in carbon chain

Nomenclature of carbon Compounds - rules used to write carbon Compounds
To write Name or Structures of compounds
Cl, N, O, S
Functional Group - is a heteroatom or group containing heteroatom which gives specific properties to compound, regardless of length and nature of carbon chain
Homologous compound - is a series of compounds in which same functional group substitutes for hydrogen in carbon chain

Nomenclature of carbon Compounds - rules used to write carbon Compounds
To write Name or Structures of compounds
 |
|
4-PART-7
4-PART-8
Light Defination: is a form of energy which travels in a straight line
Reflection Of Light , Angle of incidence( ∠i) and angle of reflection ( ∠r) Defination
Laws of reflection
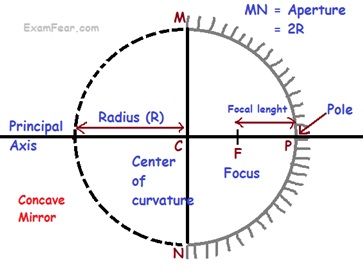
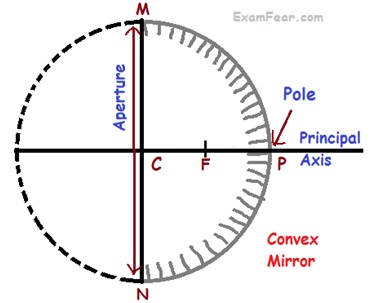
Spherical mirrors: mirrors whose reflecting surface is curved, are called spherical mirrors
Spherical mirrors are of 2 types
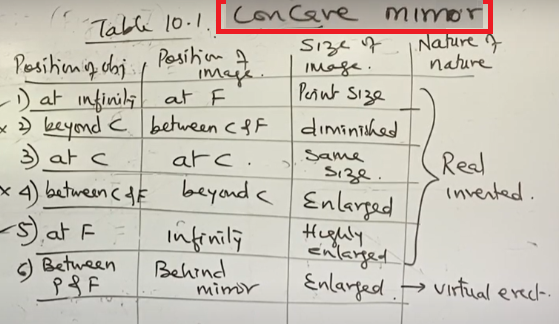
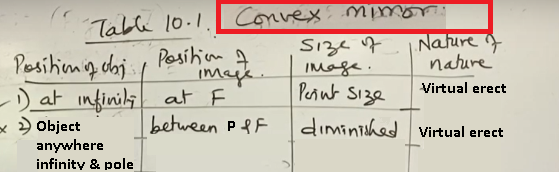
 Difference between Concave mirror and Convex mirror
Difference between Concave mirror and Convex mirror
Reflection Of Light , Angle of incidence( ∠i) and angle of reflection ( ∠r) Defination
Laws of reflection
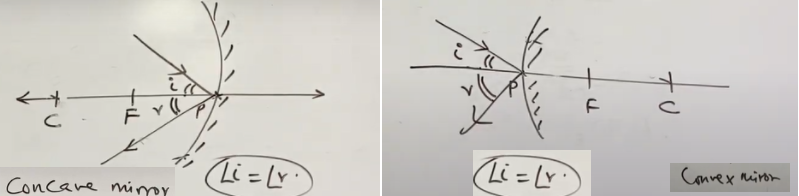
- ∠i = ∠r
- The incident ray, the reflected ray and the normal to the mirror at the point of incidence, all lie at the same plane.
- Virtual and erect
- Size of image = size of object
- Size of image = size of object
- Lateral inversion (Left side of the image becomes right side of object)
Spherical mirrors: mirrors whose reflecting surface is curved, are called spherical mirrors
Spherical mirrors are of 2 types
- Concave Mirrors
- Convex Mirrors
- Pole (P)
- Centre of curvature (C)
- Radius of curvature (R)
- Principal axis
- Principal focus (F)
- Of concave mirror
- Of convex mirror
- Focal length (f)
- Relation between Radius of curvature and focal length R = 2f
- Aperture is the diameter of reflecting surface

 Difference between Concave mirror and Convex mirror
Difference between Concave mirror and Convex mirror 10-PART-1
Rules to draw ray diagrams:
1) A ray parallel to principal axis, after reflection
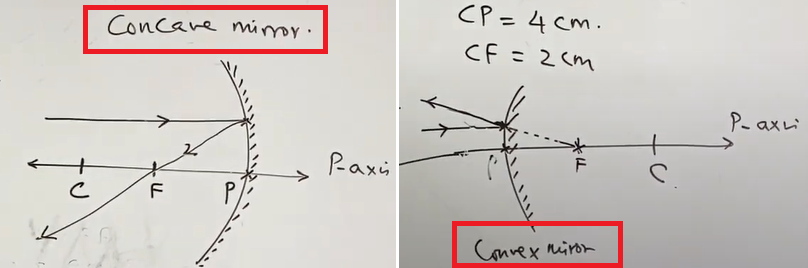
2) A ray passing through principal focus
NOTE: Principal axis is considered to be perpendicular (normal) to spherical mirror at its pole
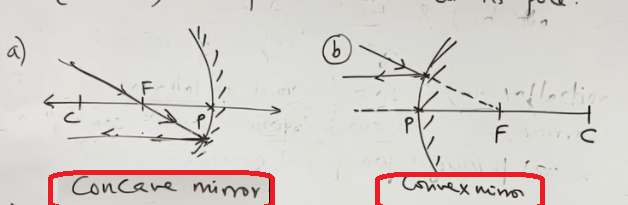
3) A ray passing through centre of curvature
Reason: This is because, the incident ray falls on mirror along the normal to reflecting surface
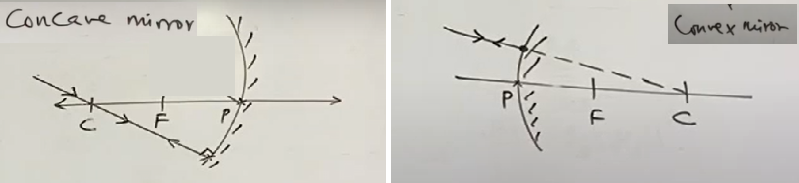
4) A ray incident obliquely to the principal axis towards the pole of concave or convex mirror is reflected obliquely such that the angle made by incident ray with principal axis and angle made by reflected ray with principal axis are equal
1) A ray parallel to principal axis, after reflection
| will pass through focus of concave mirror | or appear to diverge from principal focus of convex mirror |

2) A ray passing through principal focus
| of concave mirror after reflection will emerge parallel to principal axis | or a ray which is directed at principal focus of convex mirror after reflection will emerge parallel to principal axis |
NOTE: Principal axis is considered to be perpendicular (normal) to spherical mirror at its pole

3) A ray passing through centre of curvature
| of concave mirror after reflection is reflected along the same path. | or directed in the direction of centre of curvature of convex mirror after reflection is reflected along the same path |
Reason: This is because, the incident ray falls on mirror along the normal to reflecting surface

4) A ray incident obliquely to the principal axis towards the pole of concave or convex mirror is reflected obliquely such that the angle made by incident ray with principal axis and angle made by reflected ray with principal axis are equal
| concave mirror ∠i = ∠r | convex mirror ∠i = ∠r |

10-PART-2
New Cartesian sign convention:
Magnification
- In this convention, Pole (P) of spherical mirror is taken as origin.
- Principal axis is taken as x-axis
- The object is always placed to left of mirror
- All distances parallel to principal axis are measured from the pole of the mirror
- All distances to the right of the origin are taken as positive values, but distances measured to the left of the origin are taken as negative value
- Distances measured perpendicular to and above the principal axis are taked as positive and distances perpendicular to and below the principal axis are taken as begative values
| 1 | = | 1 + | 1 | Where f- focal length, V image distance from pole, u - object distance from pole- |
| f | v | u |
Magnification
- It is expressed as the ratio of height of image to height of object
| m = | h! | = | -v | Where h! - height of image, h - height of object |
| h | u |
10-PART-4
Sign Convention Revision:
Focal length(f)
Focal length(f)
- Focal length(f) of concave mirror is -ve
- Focal length(f) of convex mirror is +ve
- If the magnification(m) is +ve, image is virtual, erect (note: virtual image is allways erect)
- If the magnification(m) is -ve, image is real, inverted (note: real images are allways inverted)
- Object distance is u is allways -ve
- If the Heigh of Image (h!) is +ve, then image is virtual, erect
- If the Heigh of Image (h!) is -ve, then image is real, inverted
- R = 2f where f is focal length
10-PART-4-NUMERICAL BASED ON PART 4
Refraction of Light Defination:
Refraction takes place when
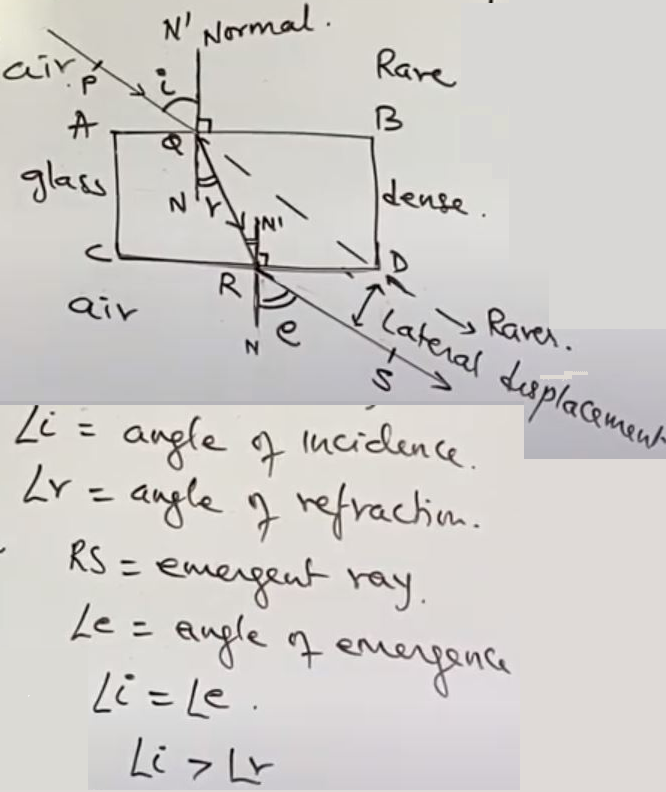
Refraction seen in every day life. (Give reasons)
Refraction revision
When ray of light travels, obliquely from
Speed of air or vaccum = 3x108 m/s
Laws of refraction:-
Refraction takes place when
- Medium are of different density
- When the ray of light is travelling Obliquely
- Medium should be transparent

Refraction seen in every day life. (Give reasons)
- In a Glass of water place a lemon. When observed lemon appears enlarge. Give Reason.
Because we are in air(rarer medium) and Lemon is in water(denser medium). When the ray of light travels from one medium to another which is transparent that time its pathway will change. This is called as refraction. due to this refraction we see the lemon enlarged - In a Glass of water place a coin. When observed coin appears to raied. Give Reason
Because we are in air(rarer medium) and Coin is in water(denser medium). When the ray of light travels from one medium to another which is transparent that time its pathway will change. This is called as refraction. due to this refraction we see coin appears to raied -
Stand Near Swimming Pool, The Swimming pool appears shallow or Floor of Swimming pool appared to be raised. Give Reasone
Because we are in air(rarer medium) and Swimming pool floor is in water(denser medium). When the ray of light travels from one medium to another which is transparent that time its pathway will change. This is called as refraction. due to this refraction we see coin appears to raied -
In a Glass of water and dip penclip/spoon partly. When Obsered it appears penclip/spoon is bend. Give Reason
Because we are in air(rarer medium) and part of penclip/spoon is in water(denser medium). When the ray of light travels from one medium to another which is transparent that time its pathway will change. This is called as refraction. due to this refraction we see penclip/spoon appears to appears as bend
Refraction revision
When ray of light travels, obliquely from
- Rarer to denser medium, it will bend towards the normal
- Denser to Rarer medium, it will bend away from normal
Speed of air or vaccum = 3x108 m/s
Laws of refraction:-
- The incident ray, the reflected ray and the Normal to the interface of 2 different media, at the point of incident, all lie in the same place
-
Snell's law: The ratio of sin of angle of incidence to the sine of angle of refraction is a constant, for the light of given color and given pair of media
Sine i = constant Where constant is
called refractive index(n)Sine r
10-PART-5
Refractive index defination:
If medium 1 is air/vaccume then
Refractive index of medium(n) is
| Sine i | = | Refractive index(n) |
| Sine r |
- Refractive index is a constant for a pair of medium
- Refractive index is also linked to the speed of light in that medium.
| n21 = | speed of light in medium 1 |
| speed of light in medium 2 |
If medium 1 is air/vaccume then
Refractive index of medium(n) is
-
nm = speed of light in air speed of light in medium -
nm = C V - Note:- When we take the medium 1 as air/vaccume, the refractive index is called absolute refractive index of that media (nm) or (n2)
-
Air has least refractive index
nair = 1.003 -
Dimand has the highest refractive index
ndimand = 2.42 - ndimand = 2.42 is highest. This means when incident ray of light enters in to dimond it's speed is becomes least and it will bend the most which will cause it to shine. Hence it is used as Jewellery
10-PART-6
Refraction by spherical lenses:
Concave lenses are diverging lenses
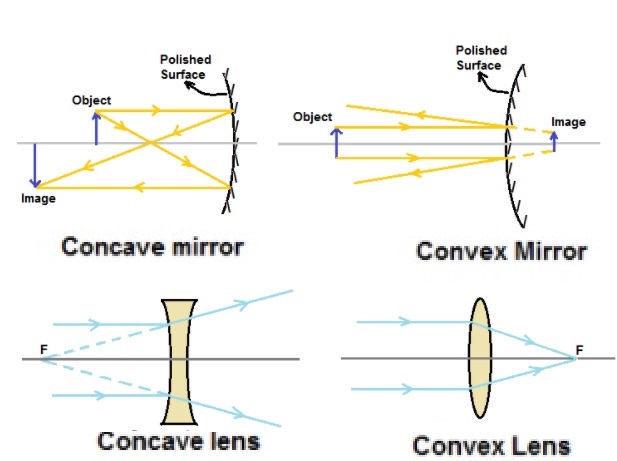
- Lense Defination
- Convex lense / Double convex lense Defination
- Concave lense / Double concave lense Defination
- Difference Between Convex lense & Concave lense
- Center of curvature
- Principal axis
- Optical center
- Aperture
- Thin lenses
- Principal Focus
- Focal Lenfth
Concave lenses are diverging lenses
- Lenses wil have two Principal foci (F1 & F2)
- Lenses wil have two Ceneter of curvature
(C1 & C2) or (2F1 & 2F2) - For thin Lenses R=2f

10-PART-7
Image Formation by lenses using Ray Diagram:
To locate the position of image we have to consider atleast two rays orignating from object.
Hence we learn the behaviour of rays in following cases.
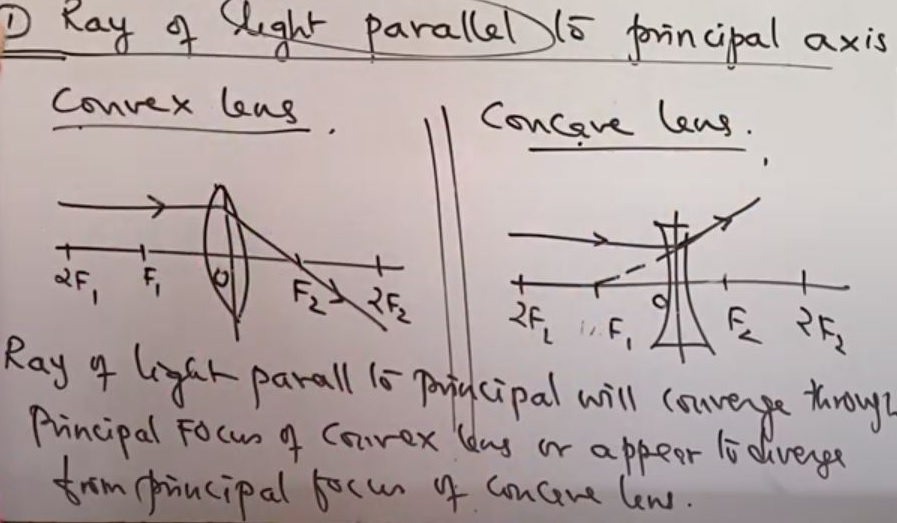

Ray Diagram for Convex lenses
To locate the position of image we have to consider atleast two rays orignating from object.
Hence we learn the behaviour of rays in following cases.



Ray Diagram for Convex lenses
- Object at infinity
- Object beyond 2F1
- Object at 2F1
- Object between 2F1 & F1
- Object at F1
- Object between F1 & O
10-PART-8
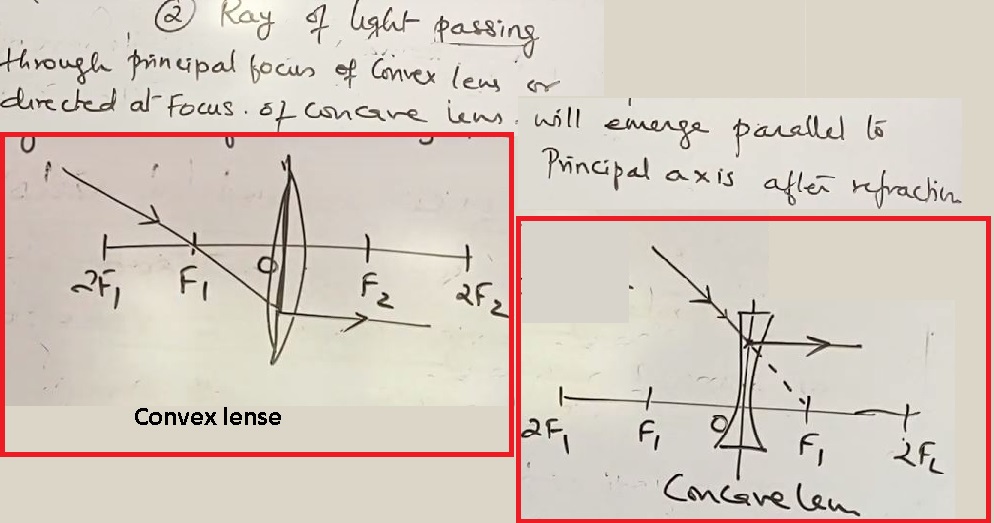
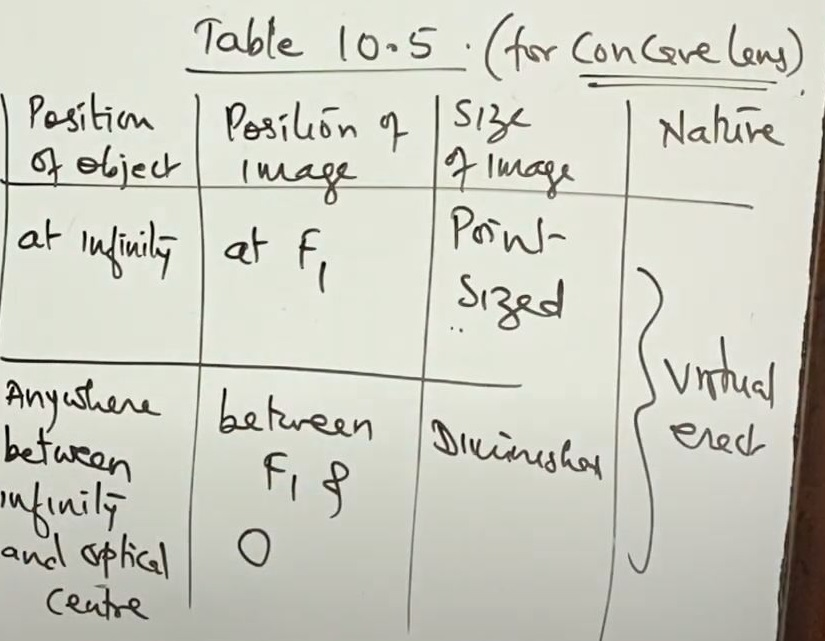
Position of image, nature & size in convex lense

Ray Diagram for Concave lenses(diverging lense)
Lense Formula
Sign Convention
Magnification
Note:
For Concave mirror & concave lense, f is -ve For Convex mirror & Convex lense, f is +ve

Ray Diagram for Concave lenses(diverging lense)
- Object at infinity
- Object anywhere between infinity & Optical Center (O)

Lense Formula
| 1 | = | 1 - | 1 | Where f- focal length, v image distance from optical center, u - object distance from optical center- |
| f | v | u | ||
Sign Convention
- For Convex lense, focal length +ve
- For Concave lense, focal length -ve
- Object distance u, is allways -ve
Magnification
- It is expressed as the ratio of height of image to height of object
- if m is +ve image is virtual
- if m is -ve image is real
| m = | h! | = | v | Where h! - height of image, h - height of object |
| h | u |
Note:
For Concave mirror & concave lense, f is -ve For Convex mirror & Convex lense, f is +ve
10-PART-9
Electric Currrent and Circuit
Numericals based on
- Circuit - is a closed path in which charges flow (electric current flows)
- Component of Circuit - blub, wire, key/switch & cell/battery
- Circuit diagram -
- Open Circuit & Close Circuit difference
-
Current = Charge I = Q Time t - SI Unit of current is - Ampere (A)
- 1 Ampere - is the electric current constituted by the flow of 1 Coulomb of charge in 1 second
- Ammmeter is an instrument that measures the current flowing in circuit
- Ammmeter is allways connected in series to device
- Electrons flow from negative terminal(-ve) to positive terminal (+ve)
-
Potential difference = Work V = W Charge Q - SI Unit of Potential difference is - Volt (V)
- SI Unit of Work is - Joule (J)
- SI Unit of Charge is - Coulomb (C)
- Voltmeter is an instrument that measures the Potential difference
- Voltmeter is allways connected in parallel to device
| Ammmeter | Voltmeter |
| It measures current in electric circuit | It measures Potential difference between two points |
| Connected in series with the device | Connected in parallel with the device |
Numericals based on
- Calculate Current(i) when Charge(Q) in Coulomb, time(t) in min is given
- Calculate Potential difference (v) when Work done(J) & Charge(Q) in Coulomb is given
12-PART-1
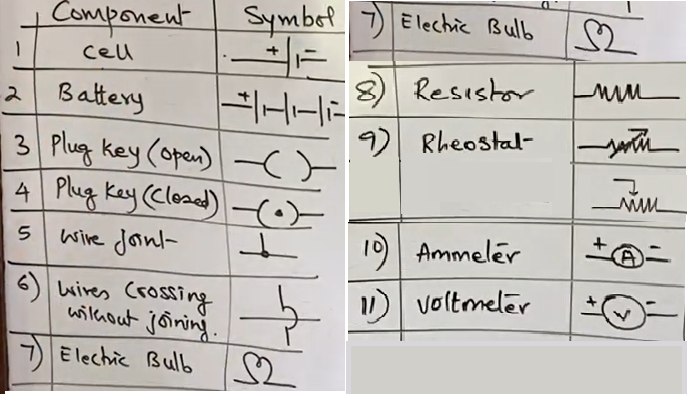
Conventional symbol used to represent the most commenely used electrical components.
Ohm's Law - Electric current flowing through a metallic wire is directly proptional to potential difference across its end, provided its temperature remains same

Ohm's Law - Electric current flowing through a metallic wire is directly proptional to potential difference across its end, provided its temperature remains same
- V ∝ I
-
V = IR OR V = R I where R is proportionality constant called Resistance - Unit of Resistance is ohm (Ω)
-
On the basis of resistance, we classify the substances into
Substances Ex Resistance Good conductors cu, ag low resistance Insulators wood very high resistance Resistor have appreciable resistance -
Rehostat defination - is a device that changes current in a device by chaning the resistance, without affecting the potential difference
ex.Regulator of fan - Changes speed of fan by changing the current entering the fan
- Rehostat is a variable resistance.
12-PART-2
Factors affecting resistance of conductor
Alloy are mixtures of metals or metal & non-metals
Alloys used in heating element is Nichrome ( Allow of Ni, Cr, Mn, Fe)
Give Reasons
- Length (l) - more the length more the resistance
R ∝ l - Area of crosss Section (A) or Thickness - more the thickness, less the resistance
R ∝ 1 A -
Conbining Length (l) & Area of crosss Section (A)
R ∝ l (length) A R = p * l (length) A p - proportionality constance called resistivity
resistivity depends on temperature & material- Metails have low resistivity
- Alloys have higher resistivity then metals
- Insulator have very high resistivity
SI unit of resistivity is ohmmeter (Ωm) - Temperature
- Material - metals like Cu, Ag have low resistance and are good conductors of electricity
wood - is a insulator
Alloy are mixtures of metals or metal & non-metals
Alloys used in heating element is Nichrome ( Allow of Ni, Cr, Mn, Fe)
Give Reasons
- Why are alloys (nichrome) used as heating element
- Why is filament of bulb made of tungston(W)
- In Which wire the current wil flow more easily - Thick wire or thin wire
- Which material are good conductors
- Why are metal wires of Cu or Al used to trnsfer electricity
- Calculate current drawn give voltage and resistance
12-PART-3
Resistance of a System of resistors - There are two ways of connecting resistors
Registers In Series (end to end)
Propertie of Series curcuit
Numericals based on registers connected in Series
Properties of Parallel curcuit
Numericals based on registers connected in Parallel
Registers In Series (end to end)
Propertie of Series curcuit
- Current flowing through each register remains same as that flowing in circuit
- The total potensial difference in the sweries circuit is equal to the sum of the potensial difference across each register
- The total resistance of series circuit is the sum of individual resistance
Rs = R1 + R2 + R3
- If one appliance fuses off the other appliances stop working because the circuit becomes open
Numericals based on registers connected in Series
- Calculate total resistance give three registers 1Ω ,2Ω & 3Ω are connected in series
Properties of Parallel curcuit
- The potensial difference across each register will remains same.
- The total current in circuit(I) is equal to the sum of the current flowing in each register
I = I1 + I2 + I3 -
The total resistance
1 = 1 1 1 Rp R1 R2 R3
- In household circuit, all appliances are connected in parallel. This is because, if one appliance fuses off than other appliance continue to operate
- Appliances drawing different current can be operated in parallel
Numericals based on registers connected in Parallel
- Calculate total resistance give three registers 1Ω ,2Ω & 3Ω are connected in Parallel
12-PART-4
Numericals based on registers in combination
- Resistance of resistance in combination ( Both Series & Parallel )
- Draw circuit diagram and calculate Total resistance, Ammeter reading, Voltmeter reading given registers values connected in series/parallel/both , bettery value
- Circuit diagram is given and asked to find the Total resistance, current in each register, Current in circuit
12-PART-5
Power of appliance - is the rate at which the electic energy is consumed
Heat
- P = VI
- SI Unit of Power is Watt(w)/KiloWatt(kw)
Heat
- H = Pt (t in seconds) or
- H = VIt (as P = VI) and (t in seconds) or
-
H = I2Rt (as V = IR by ohms law) and (t in seconds) - SI Unit of Hear is Joule(J)
- E = Pt (t in hour)
- SI Unit of Electric Energy wh(watthour)/ kwh(KiloWatt)
- Electric toaster, Electric geyser, electric kettle are based on Joule\'s law of heating
- In Bulb, electric energy is converted to heat & light
- Fuse is a safty device in circuit which operates on Joule\'s law of heating
- Fuse will protect circuit and appliances from damage when unduly high current flows in circuit
- Fuse is connected in series with appliances & placed before the appliance
12-PART-6
Numericals based on Heat Generated
- Find Heat Generated Given Charge in Coulomb & time in hr
- Find Heat Generated Given Resistance in Ω & time in sec
- Find Potential difference Given Heat Produced in Joule & Register in Ω
- Find Power Given Potential difference in volt & Current in Ampere
- Find Cost of energy for 30 days Given Power in watt, time in hr, Rs 3 Per unit(kwh)
- Find Power & Elecrtical energy Given Current in Amp, Voltage , Time in hr
12-PART-7
13-PART-3
13-PART-4
13-PART-5
13-PART-6
13-PART-7
13-PART-8